Abstract
Background:
Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate (PDC) that utilizes peptidases and esterases to release hydrophilic metabolites with alkylating activity inside tumor cells. Melflufen + dexamethasone (dex) showed superior progression-free survival (PFS; hazard ratio [HR] 0.79 [95% CI, 0.64-0.98]) vs pomalidomide (pom) + dex in relapsed/refractory multiple myeloma (RRMM) in the primary analysis of OCEAN (data cutoff date, Feb 3, 2021; Schjesvold, Lancet Hematol. 2022;9(2):e98-e110). Most patients with MM will be exposed to alkylators throughout their disease course as part of a multidrug regimen or as high-dose melphalan (140-200 mg/m2) conditioning prior to an autologous stem cell transplant (ASCT). Previous reports showed melflufen + dex is active in RRMM refractory to prior alkylator therapy and safety was not impacted (Rodriguez-Otero, et al. ASCO2021. Poster 8048; Sonneveld, et al. Blood. 2021;138(S1):4779). This updated analysis evaluated patients refractory to prior alkylators in OCEAN.
Methods:
Eligible patients with RRMM had received 2-4 prior lines of therapy (LoTs), including lenalidomide (len) and a proteasome inhibitor, and were refractory to len and to the last LoT. Patients were randomized 1:1 to receive 28-d cycles of melflufen 40 mg intravenously (d1) or pom 4 mg orally (PO; daily, d1 to 21); all patients received dex 40 mg (20 mg if aged ≥75 y) PO on d1, 8, 15, and 22. The primary endpoint was PFS (data cutoff date Feb 3, 2021). Key secondary endpoints were overall survival (OS; data cutoff date Feb 3, 2022), overall response rate (ORR), and safety. Refractoriness was defined as disease that failed to achieve a minimal response or progressed while on primary or salvage therapy or within 60 d of last dose. In patients who had or had not received a prior ASCT, efficacy was analyzed in subgroups refractory to prior alkylators.
Results:
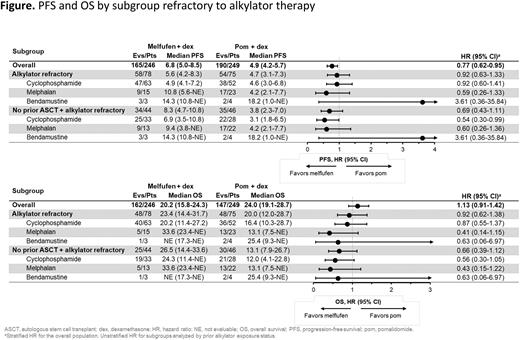
Of 246 patients randomized to melflufen and 249 randomized to pom, 78 (32%) and 75 (30%) were refractory to prior alkylators, respectively. Patients refractory to prior alkylators had received cyclophosphamide (melflufen, 81%; pom, 69%), standard dose melphalan (<140 mg/m2; melflufen, 19%; pom, 31%), and/or bendamustine (melflufen, 4%; pom, 5%). Of patients randomized to melflufen or pom, 125 (51%) and 120 (48%) had received a prior ASCT, and 121 (49%) and 129 (52%) had not received a prior ASCT, respectively.
In patients refractory to prior alkylators regardless of ASCT status, melflufen compared with pom was associated with prolonged PFS (5.6 months [95% CI, 4.2-8.3] vs 4.7 months [95% CI, 3.1-7.3]; HR, 0.92 [95% CI, 0.63-1.33]) and OS (23.4 months [14.4-31.7] vs 20.0 months [12.0-28.7]; HR, 0.92 [95% CI, 0.62-1.38]) with similar results for all alkylating agents (Figure). ORR was 24.4% for melflufen and 28.0% for pom in patients refractory to prior alkylators.
Among patients with disease refractory to prior alkylators who had not received a previous ASCT (melflufen, n=44; pom, n=46), melflufen was associated with prolonged PFS (HR, 0.69 [95% CI, 0.43-1.11]) and OS (HR, 0.66 [95% CI, 0.39-1.12]), and longer median treatment duration (8.1 months [1.0-37.8] vs 3.7 months [0.1-30.9]) than pom. In these patients, melflufen was also associated with numerically higher ORR (29.5% vs 23.9%) than pom overall and in patients refractory to melphalan (30.8% vs 22.7%) and cyclophosphamide (30.3% vs 17.9%). The safety profile of melflufen + dex in patients with disease refractory to prior alkylators was consistent with previous reports.
Conclusion:
Melflufen + dex was associated with higher PFS and OS compared with pom + dex in patients with RRMM who had disease refractory to prior alkylators, with no new safety signals identified in the patient population. These results suggest that melflufen is safe and effective in patients who are alkylator-refractory, regardless of whether they received a prior ASCT or not.
Disclosures
Schjesvold:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Targovax: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Skylite DX: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ludwig:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Consultancy, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AMGEN: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Meyers: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Consultancy; Pfizer: Consultancy, Speakers Bureau. Mateos:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS-Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Abdulhaq:Genentech: Membership on an entity's Board of Directors or advisory committees; Jansen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Speakers Bureau; Oncopeptides: Speakers Bureau; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding. Norin:Oncopeptides AB: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Thuresson:Oncopeptides: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Bakker:Oncopeptides: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Richardson:Takeda, Celgene, and GSK: Honoraria; Secura Bio: Consultancy; Takeda: Research Funding; Celgene/BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Sanofi: Consultancy; Oncopeptides: Consultancy, Research Funding; Takeda, Abbvie, GSK, and Celgene: Consultancy; Regeneron: Consultancy; GlaxoSmithKline: Consultancy; AstraZeneca: Consultancy; Protocol Intelligence: Consultancy; Takeda and GSK: Other: Travel expenses from Takeda and GSK. Sonneveld:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding.
OffLabel Disclosure:
This abstract includes a subgroup analysis of a phase 3 investigational study of melflufen in patients with RRMM refractory to lenalidomide.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal